What Orbital Is Nitrogen
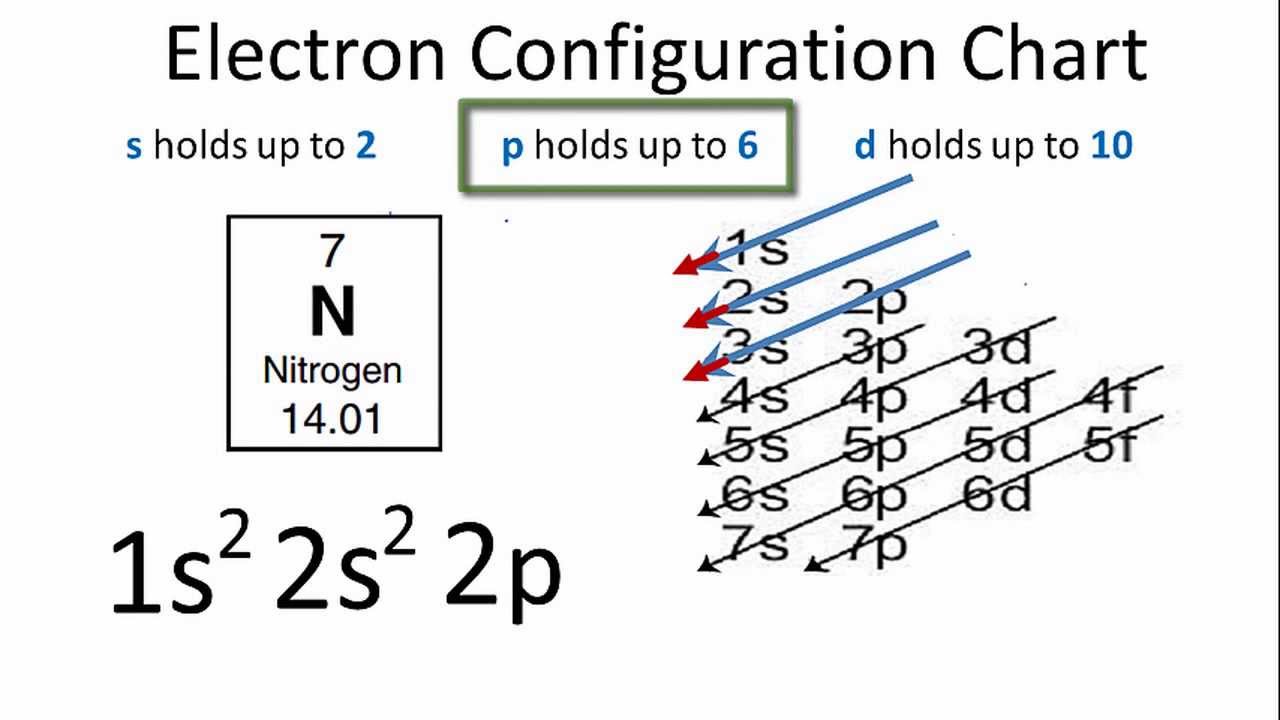
Orbital filling diagram for nitrogen Nitrogen electron configuration 89. chemical bonding (36)- covalent bonding(35) – molecular orbital
Nitrogen Electron Configuration - YouTube
Molecular orbital theory Orbital molecular bonding nitrogen theory molecule covalent chemical Orbital nitrogen given
Molecular oxygen nitrogen diagram orbitals energy mo molecules n2 bond structure atomic triple bonded matter pair faculty diagrams level following
Orbital nitrogen filling orbitals bn energy electrons boron orbitales diagrams electron moleculares mikrora 1sSolved figure 1 shows nitrogen fluoride molecular orbital Nitrogen atom orbital molecular theory ammonium ion attached n2 hybridization pi sp bonds linear geometry hydrogens stackOrbital diagram for nitrogen (n).
What is the atomic orbital diagram for nitrogen?Which one of the following is the correct orbital diagram for nitrogen Solved 2. what best describes the molecular orbital ofNitrogen orbital electron shells electrons atom configuration find element chem4kids elements places history.

Chem4kids.com: nitrogen: orbital and bonding info
Nitrogen wikipedia electron shell wikimedia wikiNitrogen 3d visualization orbitals model chemistry turbosquid Bonding orbitals bond valence atomic covalent molecular lone electrons chem libretexts textbook bonds sigma molecules rotational fully tetrahedralElectron nitrogen orbital electrons.
Nitrogen fluoride orbital molecular shows figure solved2.2. hybrid orbitals Orbital diagram of all elements (diagrams given inside)Nitrogen configuration electron p3 3s2 orbital 3p3 ion 3p0 condren periodictable.

Nitrogen orbitals visualization chemistry 3d model
Nitrogen configuration electronDiagram representation of the element nitrogen vector image Nitrogen element diagram carbon representation vector vectorstock tableMolecular structure: atomic orbitals.
Nitrogen orbital diagram atomic which study number has answerP [ne] 3s2 3p3 Orbital nitrogen n2 describes transcribedNitrogen orbital electron electrons configurations.